The word “isotope” refers to atoms that have the same number of protons and a differing number of neutrons. The number of protons, as well as electrons, in an atom control the chemical behavior of that atom, while the number of neutrons just make the atom heavier or lighter. In most active processes the weight of the atoms involved in the reaction will have a direct result on the weight of the product. For example if you were a baggage handler at an airport and you had to load 20 bags onto a plane as fast as possible (with a huge pile to choose from) you would most likely choose the 20 lightest bags. Thus the average weight of the resulting pile in the plane would be lighter than the average weight of the initial pile. Also the remaining pile on the ground (the delayed baggage) will be heavier, on average, than the average weight of the initial pile. Why? This is the same for active chemical processes such as diffusion across a boundary, bacterial metabolism, or simple equilibrium isotope exchange. By examining isotopic values of samples we can begin to understand the history of a sample, and infer what active processes it has undergone. Furthermore, isotopes can help us identify the sources contributing to a sample that represents a mixture, much like a police detective uses fingerprints to identify suspects.
 |
For Lost City the stable carbon isotopes of CO
2 (carbon dioxide) and CH
4 (methane) are of great interest because they can tell us not only about rock-fluid processes, but also about what types of metabolic activity may be associated with organisms that live on and within the chimneys. The stable carbon isotopes are 13C and 12C, and are called “stable” because they do not radioactively decay like 14C. On average, there is one 13C atom for every 100 carbon atoms, and thus 13C is somewhat easier to measure than 14C (one in every trillion carbon atoms). To measure 13C and 12C of the fluids we use a
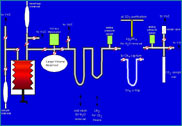
low energy mass spectrometer, an instrument the size of two washing machines, at the Stable Isotope Laboratory at the University of Washington. The difficult part of the measurement is actually all the preparation that goes into readying the sample to be measured. The sample must be a pure gas (only CO
2 or only methane), and must represent all the gas that was initially present in the fluid sample. In order to achieve these requirements we use a vacuum line and various tricks based on the freezing points of the different gases (e.g. liquid nitrogen freezes CO
2 but not methane, dry ice freezes water vapor but not CO
2).